Product
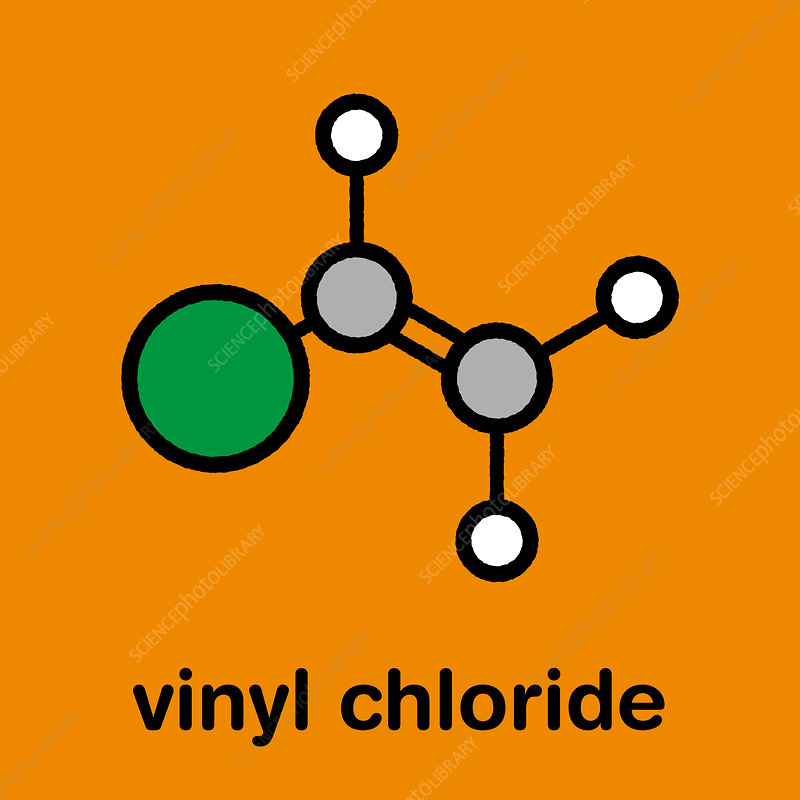
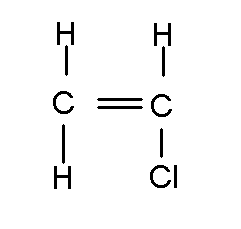
Vinyl Chloride
Introduction
Vinyl chloride, also known as chloroethene, is an organic compound with the chemical formula C2H3Cl. It is a colorless gas with a slightly sweet odor and is a key intermediate in the production of polyvinyl chloride (PVC), one of the most widely used plastics in the world. Vinyl chloride is highly flammable and has industrial importance due to its polymerization into PVC, which has a wide range of applications in the construction, automotive, and packaging industries.
-
Preparation using traditional method
The traditional method for the preparation of vinyl chloride involves the chlorination of ethylene, a process known as the oxychlorination of ethylene. In this method, ethylene gas is reacted with hydrogen chloride and oxygen in the presence of a catalyst. The reaction proceeds through several steps, ultimately leading to the formation of vinyl chloride. This method has been used for many years in industrial settings but has some disadvantages.
-
Reaction
The traditional method begins with the reaction of ethylene (C2H4) with hydrogen chloride (HCl) and oxygen (O2) in the presence of a catalyst, often a copper chloride compound. This reaction leads to the formation of vinyl chloride (C2H3Cl) and water (H2O). The chemical equations for this process are quite complex, involving multiple intermediate steps, but it ultimately results in the production of vinyl chloride, which can be separated and purified.
-
Disadvantages of traditional method
The traditional method of preparing vinyl chloride using oxychlorination has several disadvantages. It is energy-intensive, requires the use of toxic chlorine gas, and can produce harmful byproducts. Additionally, the process may not be environmentally friendly, and there is a need for careful management of byproducts and waste. These issues have led to a search for greener alternatives for vinyl chloride production.
-
Preparation using Green method
A greener and more sustainable method for vinyl chloride production involves using bio-based feedstocks and renewable resources. One such method utilizes bioethanol, which is derived from agricultural products like corn or sugarcane, as a starting material. Bioethanol can be dehydrated to produce ethylene, which is then chlorinated to form vinyl chloride. This approach reduces the environmental impact and reliance on fossil fuels.
-
Reaction
In the green method, bioethanol is first dehydrated to produce ethylene. This ethylene can then undergo chlorination using a more environmentally friendly process, typically involving the use of hydrogen chloride or other green chlorinating agents. The reaction leads to the formation of vinyl chloride with fewer harmful byproducts and reduced environmental impact.
-
Advantages of Green method
The green method for vinyl chloride production offers several advantages over the traditional method. It relies on renewable and sustainable resources, reduces the use of toxic chemicals, and minimizes environmental pollution. It aligns with the principles of green chemistry and contributes to a more environmentally friendly and sustainable chemical industry.
-
Applications
Vinyl chloride is primarily used in the production of polyvinyl chloride (PVC) resin, one of the most versatile and widely used plastics globally. PVC has an extensive range of applications, including pipes, cable insulation, vinyl siding for buildings, medical devices, clothing, and packaging materials. Vinyl chloride's polymerization into PVC imparts desirable properties such as durability, chemical resistance, and flexibility, making it an essential material in various industries. Its applications continue to expand as new formulations and uses for PVC are developed.