Product
Sulphuric Acid
Introduction
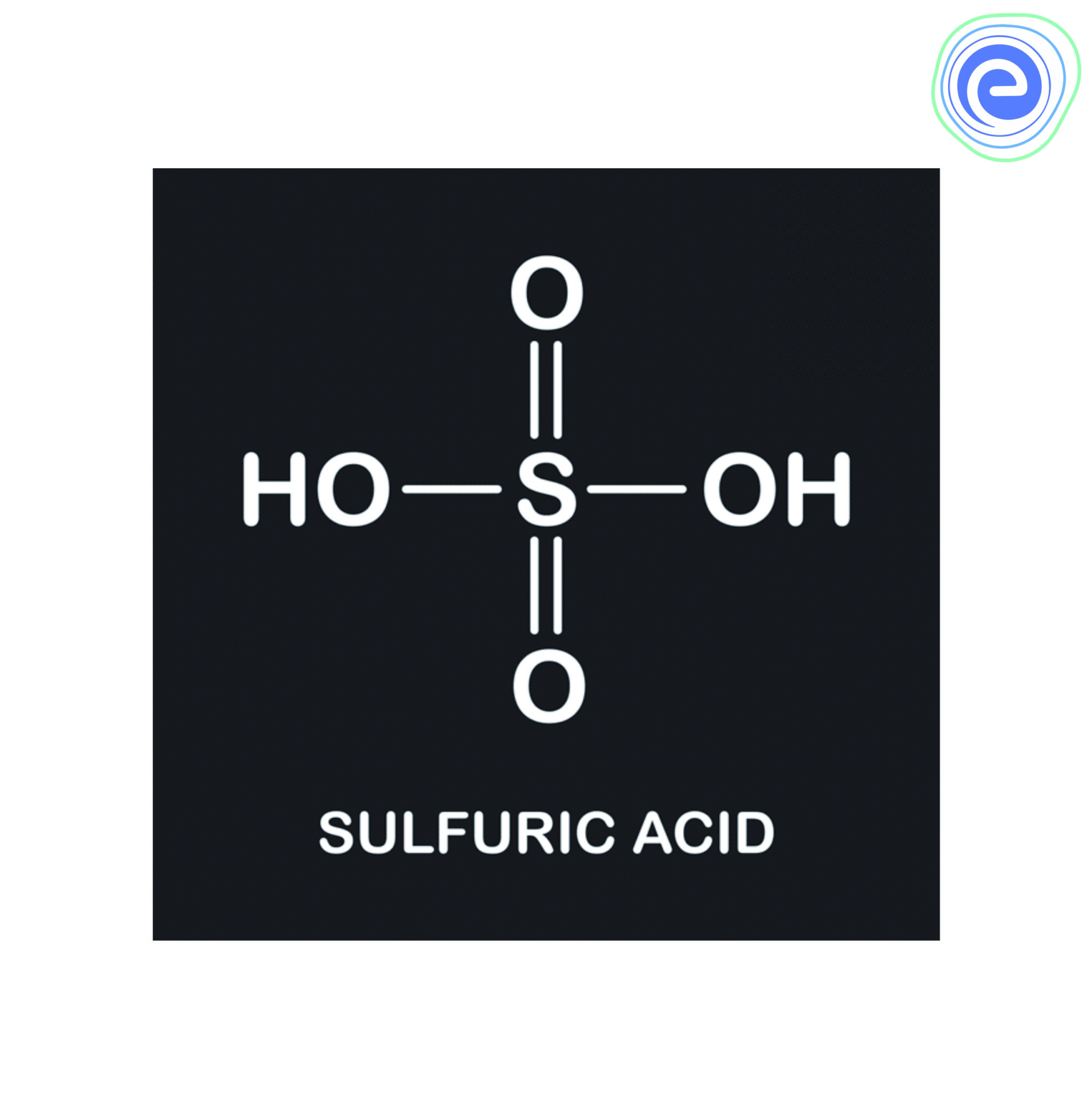
Sulfuric acid, chemical formula H2SO4, is a strong, highly corrosive mineral acid with a wide range of industrial applications. It is often referred to as the "king of chemicals" due to its importance in various chemical processes. Sulfuric acid is a colorless, odorless liquid that is soluble in water and known for its ability to dehydrate substances and act as a powerful acid in chemical reactions.
-
Preparation using traditional method
The traditional method for preparing sulfuric acid involves a two-step process. First, sulfur dioxide (SO2) is produced by burning sulfur or roasting sulfide ores, which is then oxidized to sulfur trioxide (SO3) using a catalyst like vanadium(V) oxide. The sulfur trioxide is then reacted with water to produce concentrated sulfuric acid. This method has been widely used for many years but is energy-intensive and can release significant amounts of sulfur dioxide, a pollutant and environmental concern.
-
Reaction
The key chemical reaction in the traditional preparation of sulfuric acid involves the combination of sulfur trioxide (SO3) with water (H2O) to produce H2SO4: SO3 + H2O → H2SO4 This reaction is highly exothermic and results in the formation of concentrated sulfuric acid.
-
Disadvantages of traditional method
The traditional method for preparing sulfuric acid has several disadvantages, including its high energy consumption and the emission of sulfur dioxide, a major air pollutant. The process also requires specialized equipment and can be expensive due to the use of vanadium(V) oxide as a catalyst. Additionally, it poses environmental and health risks due to the release of toxic byproducts.
-
Preparation using Green method
Green methods for preparing sulfuric acid focus on reducing the environmental impact and energy consumption. One green approach involves the catalytic oxidation of sulfur dioxide to sulfur trioxide using environmentally friendly catalysts like vanadium phosphate. This reduces the environmental footprint of the production process.
-
Reaction
In green methods, the chemical reaction to produce sulfuric acid remains the same, with sulfur trioxide reacting with water to form H2SO4. However, the green approach emphasizes the use of more sustainable and environmentally friendly catalysts in the oxidation step to produce sulfur trioxide.
-
Advantages of Green method
The green method for preparing sulfuric acid offers several advantages, including reduced energy consumption, lower environmental impact, and the use of more sustainable catalysts. This approach aligns with modern environmental and sustainability goals by minimizing harmful emissions and optimizing resource utilization.
-
Applications
Sulfuric acid is a versatile chemical with numerous applications across various industries. It is used in the production of fertilizers, detergents, and chemicals, as well as in the petroleum and automotive industries. Sulfuric acid is also a crucial component in lead-acid batteries and serves as an electrolyte in these batteries. Additionally, it finds application in the mining industry for extracting metals from ores and in various laboratory and analytical procedures.