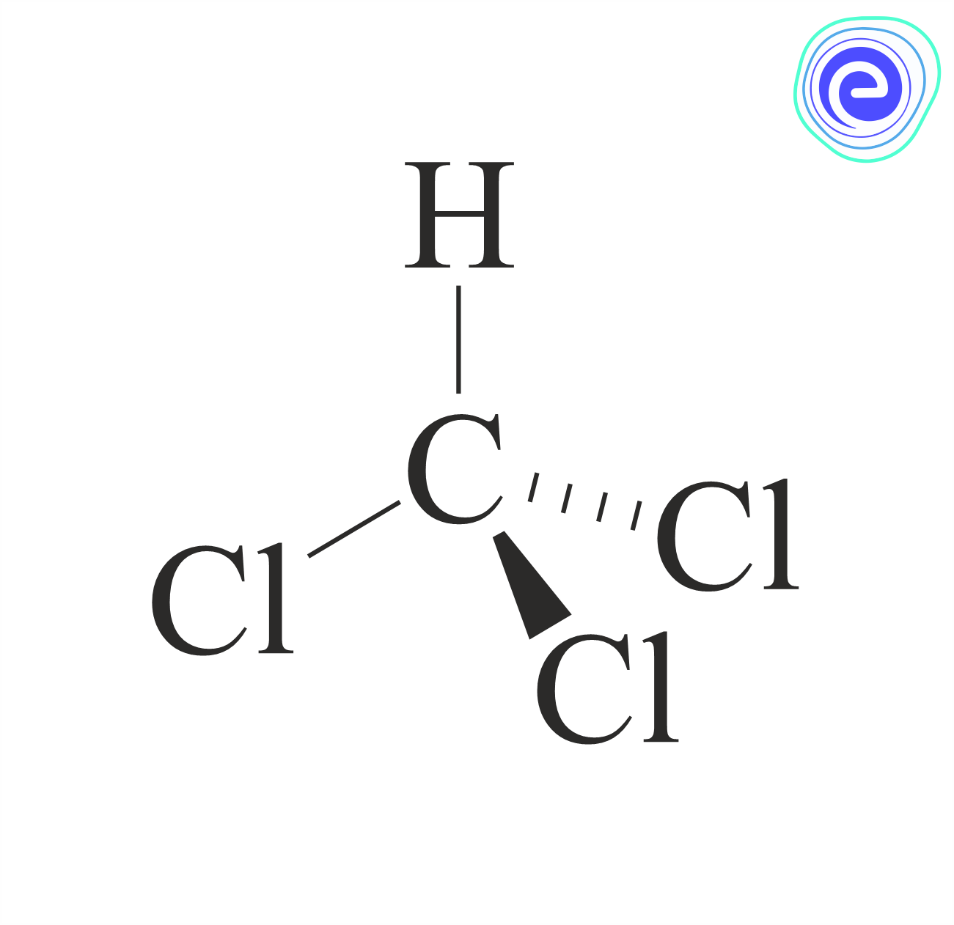
Product
Chloroform
Introduction
Chloroform, also known as trichloromethane, is a chemical compound with the molecular formula CHCl3. It is a colorless, sweet-smelling organic compound that gained notoriety as an anesthetic in the 19th century but is now primarily recognized as a hazardous chemical due to its toxic and carcinogenic properties.
-
Preparation using traditional method
Chloroform was historically prepared through the reaction of ethanol and sodium hypochlorite, followed by distillation. This process, although effective, has fallen out of favor due to safety concerns and environmental issues associated with the use of chlorine-based reagents.
-
Reaction
The traditional preparation of chloroform involves the chlorination of ethanol with sodium hypochlorite (bleach) in the presence of a strong base like sodium hydroxide. This reaction results in the formation of chloroform and other byproducts. CH3CH2OH + 3NaOCl + 3NaOH → CHCl3 + 3NaCl + 3H2O
-
Disadvantages of traditional method
The primary disadvantage of the traditional method of chloroform preparation is the production of hazardous byproducts, such as sodium chloride and water, as well as the environmental concerns associated with the use of chlorine-based reagents. Additionally, the process can be dangerous due to the potential release of toxic chlorine gas.
-
Preparation using Green method
To address the environmental and safety concerns associated with the traditional method, a more environmentally friendly or "green" approach to chloroform synthesis involves using alternative reagents and methods. One such method is the direct chlorination of methane or the reaction of acetone with a mild chlorinating agent.
-
Reaction
In the green method, chloroform can be prepared through the direct chlorination of methane (CH4) using ultraviolet (UV) light as a catalyst, or by reacting acetone (CH3COCH3) with a mild chlorinating agent like chlorine or bromine. These processes generate chloroform with fewer harmful byproducts and reduced environmental impact.
-
Advantages of Green method
The main advantage of the green method for chloroform preparation is the reduction in hazardous byproducts and the use of milder and more environmentally friendly reagents. This approach minimizes the environmental impact and enhances the safety of the synthesis process.
-
Applications
Chloroform, despite its hazardous nature, has found applications in the past as an anesthetic, solvent, and for various industrial uses. However, its use has significantly declined due to health and environmental concerns. Today, it is primarily utilized as a reagent in chemical laboratories for specific reactions and is not recommended for general applications due to its toxicity.